Syringocystadenoma papilliferum in an unusual location beyond the head and neck region: A case report and review of literature
Published Web Location
https://doi.org/10.5070/D39wk364xhMain Content
Syringocystadenoma papilliferum in an unusual location beyond the head and neck region: A case report and review of literature
Felix Boon-Bin Yap MD MRCP1, Bang Rom Lee MBBS MMed2, Roshidah Baba MBBS FRCP1
Dermatology Online Journal 16 (10): 4
1. Department of Dermatology, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia. woodzlamp@yahoo.com2. Department of Pathology, Faculty of Medicine and Health Science, Universiti Putra Malaysia, Selangor Darul Ehsan, Malaysia
Abstract
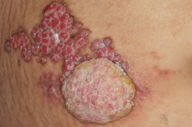
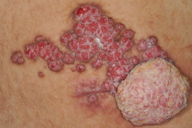
A case of syringocystadenoma papilliferum with multiple papulonodules in a linear fashion located in an unusual location of the right lower abdomen is presented. The presence of a large tumor at the inferior pole raised the suspicion of malignant transformation and the presence of discharge from the lesions raised the possibility of necrosis. However, histopathological examination showed the classical features of syringocystadenoma papilliferum without malignant transformation or tumor necrosis. The patient refused to undergo surgical excision of the nodules and subsequently was lost to follow-up. This case illustrates the atypical location of a rare disease and adds to the differential diagnosis of linear verrucous lesions on the abdomen. Review of all the cases with syringocystadenoma papilliferum outside the head and neck region in the English literature showed only one case of syringocystadenoma papilliferum arising on the abdomen; our patient is the second reported case with the unique feature of linear arrangement of lesions.
Introduction
Syringocystadenoma papilliferum is a benign hamartomatous adnexal tumor. The histogenesis of this rare neoplasm is still unclear. It is theorized that syringocystadenoma papilliferum arises from the pluripotent cells of apocrine lineage [1]. The tumor may occur de novo or within a nevus sebaceus [2]. Most patients present with a solitary lesion in the head and neck region at birth or in early childhood [2, 3]. Presentation with multiple lesions is rare; those arising outside the head and neck region are even more uncommon. We present a case of syringocystadenoma papilliferum with multiple verrucous lesions, arranged in a linear fashion and located in the unusual site of the lower abdomen.
Case report
A 40-year-old woman presented with multiple nontender and nonpruritic verrucous papules and nodules on the right lower abdomen. She had these lesions since birth and they had gradually increased in number and size over the years. Currently, she presented with a 6-month history of progressive enlargement of the eruption, associated with foul smelling discharge. She admitted to have applied traditional topical treatment to the lesions. There were no associated systemic symptoms. She also had type 2 diabetes mellitus, for which she was taking oral metformin 750 mg twice daily. In addition, she had hypertension for which she was taking oral amlodipine 5 mg daily for the past 2 years.
On examination, multiple erythematous papules and nodules were observed on the right lower abdomen arranged in a linear fashion (Figures 1 and 2). These papules and nodules measured up to 1.5 cm in diameter with the largest on the inferior pole measuring 5 cm in diameter. Erosion was seen on the surface of some of the lesions. There was also yellowish creamy foul smelling discharge.
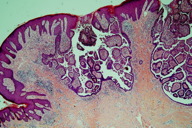
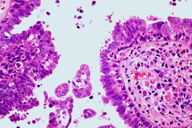
Two incisional skin biopsies were performed, one on the largest tumor and another on a small nodule. The histopathological examinations of both biopsies showed the presence of multiple cystic, papillary, and ductal invaginations extending into the dermis. These invaginations were connected to the epidermis, which showed acanthosis and papillomatosis (Figure 3). Double layers of cells consisting of an inner layer of cuboidal cells and an outer luminal layer of tall columnar cells were seen lining the invaginations (Figure 4). Decapitation secretion was seen in the luminal layer (Figure 4). The connective tissue core was filled with plasma cells. There were no histological features of nevus sebaceus or epidermal nevus. The histological findings were consistent with the diagnosis of syringocystadenoma papilliferum.
Culture of the discharge from the lesions grew Pseudomonas aeruginosa and she was treated with oral ciprofloxacin and intravenous amikacin for 2 weeks. The patient was later seen in the combined dermatology-plastic surgery consultation and was offered surgical excision with skin grafting. However, she refused the surgical treatment and instead opted for traditional Malay therapy. She also refused to be patch tested to the traditional topical medications she had used for the skin lesions. She subsequently was lost to follow-up.
Discussion
Since the first description by Stokes in 1917 under the term nevus syringadenomatosus papilliferus, syringocystadenoma papilliferum has been increasingly reported in the English literature [1]. The majority of the reported cases had lesions in the head and neck region. Mammino et al. reviewed 145 cases with syringocystadenoma papilliferum and found 108 cases (75.0%) occurring on the head and neck region, 29 cases (20.0%) on the trunk, and 8 cases (5.0%) on the extremities [2]. However, over the past two decades there are increasing reports of syringocystadenoma papilliferum occurring in unusual locations outside the head and neck region.
We reviewed the English literature for cases of syringocystadenoma papilliferum outside the head and neck region and found 69 cases with such characteristics (Table 1). There were 67 cases with syringocystadenoma papilliferum in one body location and 2 cases with the tumor in 2 different body locations. There were 38 (53.5%) syringocystadenoma papilliferum tumors seen on the trunk, 24 (33.8%) on the extremities, and 9 (12.5%) on the genitalia. Of the 38 neoplasms on the trunk, 3 tumors were reported on the breast, 2 on the back, 1 on the abdomen, 1 on the chest, and 31 on the trunk (not specified). On the extremities, 9 were reported on the lower limbs, 3 on the upper limbs, 2 on the buttocks, and 10 on the limbs (not specified). On the genitalia, 5 syringocystadenoma papilliferum were seen on the vulva and 4 on the scrotum. The sizes of the skin lesions ranged from 5 to 160 mm. There were 13 cases (18.8%) that had the lesions since birth. Associated lesions were seen in 12 cases (17.3%). These associated lesions included viral warts, nevus sebaceus, linear nevus verrucosus, nevus comedonicus, apocrine poroma, apocrine hidrocystoma, tubulopapillary hidradenoma, hidradenoma papilliferum, papillary eccrine adenoma, verrucous carcinoma, and syringocystadenocarcinoma papilliferum.
To date, there is only one reported case of syringocystadenoma papilliferum arising on the abdomen [10]. That 65-year-old Japanese man had syringocystadenoma papilliferum associated with apocrine poroma for 4 years prior to his presentation. In contrast our patient had the lesions since birth with no associated skin lesion. Moreover, there were multiple lesions arranged in a linear fashion in our patient compared to a solitary ulcerated lesion in the elderly man.
Syringocystadenoma papilliferum presents as a brownish or erythematous papule, nodule, or tumor of varying sizes. The surface can be smooth, flat, papillomatous, or verrucous [2]. Most patients present with a solitary lesion. However, if there are multiple papules, they tend to be arranged in a linear configuration [3]. The latter lesions are usually associated with nevus sebaceus [15]. Syringocystadenoma papilliferum is also associated with apocrine poroma, apocrine acrosyringeal keratosis, apocrine hidrocystoma, tubular apocrine adenoma, papillary eccrine adenoma, eccrine nevus, tubulopapillary hidroadenoma, hidroadenoma papilliferum, poroma folliculare, linear nevus verrucosa, atypical fibroxanthoma, clear cell syringoma, basal cell epithelioma, sebaceous epithelioma, trichoepithelioma, verruca vulgaris, and nevus comedonicus [10, 17, 20, 23, 26, 30-38]. Fujita et al noted that the most common associated lesion is nevus sebaceus (31.7%) followed by basal cell epithelioma (10.3%), sebaceous epithelioma (3.2%), apocrine hidrocystoma (3.2%), trichoepithelioma (1.6%), and eccrine spiradenoma (0.8%) [34].
Clinically, the differential diagnoses of these linear lesions include linear epidermal nevus, nevus comedonicus, basaloid follicular hamartoma, cylindroma, and eccrine nevus [39]. Other possible diagnoses include verrucous carcinoma, viral warts, pyogenic granuloma, subcutaneous fungal infection, tuberculosis verrucosa cutis, and giant lymphangioma [5].
Histopathological examination of syringocystadenoma papilliferum frequently shows multiple cystic invaginations in a background of fibrous tissue. The upper regions of the cystic invaginations are commonly lined by keratinizing squamous epithelial cells whereas the lower regions, which contain papillary projections, are lined by glandular epithelium. These papillary projections are the most characteristic pattern [2]. The glandular epithelium is lined by two layers of cells namely the tall columnar cells at the luminal surface and the cuboidal cells at the base. Decapitation secretion or “apical snouts” are often seen at the luminal surface. Another diagnostic feature of this neoplasm is the presence of mononuclear inflammatory infiltrates consisting of mainly plasma cells in the fibrous tissue of the papillary projections [2]. Positive staining in the luminal cells for alcian blue, colloidal iron, and periodic acid-Schiff (PAS), which is diastase resistant, favor the apocrine differentiation of this tumor [40, 41]. Moreover, the positivity for immunohistochemical staining of gross cystic disease fluid protein 15 (GCDFP-15; BRST 2), Leu-M1 antigen (CD 15), lysozymes, carcinoembryonic antigen (CEA), and epithelial membrane antigen (EMA) further support the theory of apocrine origin [4, 15, 42, 43]. Nevertheless, there are also some studies that showed an eccrine differentiation of the neoplasm [44, 45]. Thus, the jury is still out on the etiology of this benign tumor.
The development of the large tumor on the inferior pole in our patient was worrisome because of the possibility of malignant transformation. Syringocystadenoma papilliferum is associated with malignant tumors such as verrucous carcinoma, basal cell carcinoma, sebaceus carcinoma and ductal carcinoma [6, 7, 8]. Syringocystadenocarcinoma papilliferum, a malignant counterpart of syringocystadenoma papilliferum, can also arise in the benign hamartoma [9]. Fortunately, there were no features to suggest malignant change in the histopathological examination of our patient.
Another interesting note in our patient was the foul smelling discharge from the syringocystadenoma papilliferum. We suspect that this was a secondary skin infection associated with the allergic contact dermatitis caused by the application of traditional topical treatment. There was also the possibility of spontaneous necrosis of the syringocystadenoma papilliferum lesions giving rise to the discharge. However, we did not detect any features of necrosis on the histopathological examination to support this theory.
The treatment of choice for syringocystadenoma papilliferum is complete surgical excision. However, carbon dioxide laser can be useful for lesions on anatomic sites not suitable for surgery [46, 47].
In conclusion, we have added another case of linear syringocystadenoma papilliferum in an unusual location beyond the head and neck region. Syringocystadenoma papilliferum must be included in the differential diagnosis of linear verrucous lesions on the abdomen.
References
1. Stokes JH. A clinico-pathologic study of an unusual cutaneous neoplasm combining nevus syringadenomatosus papilliferus and a granuloma. J Cutan Dis. 1917; 35: 411-9.2. Mammino J, Vidmar D. Syringocystadenoma papilliferum. Int J Dermatol. 1991; 30(11): 763-6. [PubMed]
3. Goldberg NS, Esterly NB. Linear papules on the neck of a child. Arch Dermatol. 1985; 121: 1198, 1201. [PubMed]
4. Xu X, Zhang G, Zeng X, Wang Q, Sun J. A case of zonal syringocystadenoma papilliferum of the axilla mimicking verruca vulgaris. Am J Dermatopathol. 2010; 32(1): 49-51. [PubMed]
5. Malhotra P, Singh A, Ramesh V. Syringocystadenoma papilliferum on the thigh: an unusual location. Indian J Dermatol Venereol Leprol. 2009; 75(2): 170-2. [PubMed]
6. Abdulla AN, Covert AA, Grantmyre JE. Scrotal syringocystadenoma papilliferum: case report. Can J Urol. 2009; 16(3): 4684-6. [PubMed]
7. Ghosh SK, Bandyopadhyay D, Chatterjee G, Bar C. Syringocystadenoma papilliferum: an unusual presentation. Pediatr Dermatol. 2009; 26(6): 758-9. [PubMed]
8. Stewart CJ. Syringocystadenoma papilliferum-like lesion of the vulva. Pathology. 2008; 40(6): 638-9. [PubMed]
9. Rammeh-Rommani S, Fezaa B, Chelbi E, Kammoun MR, Ben Jilani SB, Zermani R. Syringocystadenoma papilliferum: report of 8 cases. Pathologica.2006; 98(3): 178-80. [PubMed]
10. Suzuki T, Ikeda H, Hamasaki Y, Hatamochi A, Yamazaki S. Syringocystadenoma papilliferum associated with apocrine poroma. J Dermatol. 2006; 33(4): 249-51. [PubMed]
11. Vaos G, Pierrakou P. Syringocystadenoma papilliferum: a rare breast tumor in a young boy. Pediatr Dev Pathol. 2006; 9(5): 381-3. [PubMed]
12. Yoshii N, Kanekura T, Setoyama M, Kanzaki T. Syringocystadenoma papilliferum: report of the first case on the lower leg. J Dermatol. 2004; 31(11): 939-42. [PubMed]
13. Townsend TC, Bowen AR, Nobuhara KK. Syringocystadenoma papilliferum: An unusual cutaneous lesion in a pediatric patient. J Pediatr. 2004; 145(1): 131-3. [PubMed]
14. Goshima J, Hara H, Okada T, Suzuki H. Syringocystadenoma papilliferum arising on the scrotum. Eur J Dermatol. 2003; 13(3): 271. [PubMed]
15. Patterson JW, Straka BF, Wick MR. Linear syringocystadenoma papilliferum of the thigh. J Am Acad Dermatol. 2001; 45(1): 139-41. [PubMed]
16. Sood A, Khanna N, Kumar R. Syringocystadenoma papilliferum at unusual sites. Indian J Dermatol Venereol Leprol. 2000; 66: 328-9.
17. Coyne JD, Fitzgibbon JF. Mixed syringocystadenoma papilliferum and papillary eccrine adenoma occurring in a scrotal condyloma. J Cutan Pathol. 2000; 27(4): 199-201. [PubMed]
18. Nowak M, Pathan A, Fatteh S, Fatteh S, Lopez J. Syringocystadenoma papilliferum of the male breast. Am J Dermatopathol. 1998; 20(4): 422-4. [PubMed]
19. Ndiaye B, Kane A, Develoux M, Dieng MT, Saccharin C. Syringocystadenoma papilliferum. A case located on the knee. Ann Dermatol Venereol. 1994; 121(4): 323-4. [PubMed]
20. Skelton HG 3rd, Smith KJ, Young D, Lupton GP. Condyloma acuminatum associated with syringocystadenoma papilliferum. Am J Dermatopathol. 1994; 16(6): 628-30. [PubMed]
21. Yamamoto T, Mamada A. Syringocystadenoma papilliferum arising on the thigh without connection to the overlying epidermis. Am J Dermatopathol. 2008; 30(1): 84-5. [PubMed]
22. de Bliek JP, Starink TM. Multiple linear syringocystadenoma papilliferum. J Eur Acad Dermatol Venereol. 1999; 12(1): 74-6. [PubMed]
23. Lee HJ, Chun EY, Kim YC, Lee MG. Nevus comedonicus with hidradenoma papilliferum and syringocystadenoma papilliferum in the female genital area. Int J Dermatol. 2002; 41(12): 933-6. [PubMed]
24. Monticciolo NL, Schmidt JD, Morgan MB. Verrucous carcinoma arising within syringocystadenoma papilliferum. Ann Clin Lab Sci. 2002; 32(4): 434-7.[PubMed]
25. Gönül M, Soylu S, Gül U, Kaya I, Albayrak L, Unal T. Linear syringocystadenoma papilliferum of the arm: a rare localization of an uncommon tumour. Acta Derm Venereol. 2008; 88(5): 528-9. [PubMed]
26. Hoekzema R, Leenarts MF, Nijhuis EW. Syringocystadenocarcinoma papilliferum in a linear nevus verrucosus. J Cutan Pathol. 2009. [Epub ahead of print] [PubMed]
27. Nabeel Al-Brahim, Dean Daya, Salem Alowami. A 64-Year-Old Woman With Vulvar Papule. Vulval syringocystadenoma papilliferum. Arch Pathol Lab Med. 2005; 129( 5): e126-e127. [PubMed]
28. Aydin, G. and S. Aydin. Syringocystadenoma papilliferum as a cutaneous mass in an unusual location: a case report. Pak J Med Sci. 2003; 19: 220-225.
29. Abdulla AN, Covert AA, Grantmyre JE. Scrotal syringocystadenoma papilliferum: case report. Can J Urol. 2009; 16(3): 4684-6. [PubMed]
30. Sardesai VR, Agarwal VM, Manwatkar PP, Gharpuray MB. Giant condyloma acuminata with syringocystadenoma papilliferum. Indian J Dermatol Venereol Leprol 2009; 75: 330. [PubMed]
31. Arias-Santiago S, Aceituno-Madera P, Aneiros-Fernandes J, Gutierrez-Salmeron MT, Naranjo-Sintes R. Syringocystadenoma papilliferum associated with apocrine hidrocystoma and verruca. Dermatol Online J 2009; 15(11): 9. [PubMed]
32. Hsu PJ, Liu CH, Huang CJ. Mixed tubulopapillary hidradenoma and syringocystadenoma papilliferum occurring as a verrucous tumor. J Cutan Pathol. 2003; 30(3): 206-10. [PubMed]
33. Kim MS, Lee JH, Lee WM, Son SJ. A case of tubular apocrine adenoma with syringocystadenoma papilliferum that developed in a nevus sebaceous. Ann Dermatol. 2010; 22(3): 319-22. [PubMed]
34. Fujita M, Kobayashi M. Syringocystadenoma papilliferum associated with poroma folliculare. J Dermatol. 1986; 13: 480-482. [PubMed]
35. Kishimoto S, Wakabayashi S, Yamamoto M, Yoda Y, Takenaka H, Yasuno H. Apocrine acrosyringeal keratosis in association with syringocystadenoma papilliferum. Br J Dermatol 2000; 142: 543-547. [PubMed]
36. Philipone E, Chen S. Unique case: syringocystadenoma papilliferum associated with an eccrine nevus. Am J Dermatopathol. 2009; 31(8): 806-7. [PubMed]
37. Tronnier M, Vogelbruch M. Atypical fibroxanthoma arising in an area of syringocystadenoma papilliferum associated with nevus sebaceous: positivity of the atypical fibroxanthoma component of CD31. Cutan Pathol. 2007; 34 (Suppl 1): 58-63. [PubMed]
38. Lin WL, Lin WC, Kuo TT, Chen CH, Hong HS. An unsual complex cutaneous adnexal tumor composed of syringocystadenoma papilliferum, apocrine hidrocystoma and clear cell syringoma. Dermatol Surg. 2007; 33(7): 876-9. [PubMed]
39. Jimenez-Acosta FJ, Redondo E, Baez O, Hernandez B. Linear unilateral basaloid follicular hamartoma. J Am Acad Dermatol 1992; 27(2 Pt 2): 316-9. [PubMed]
40. Lever WF, Schaumburg-Lever G. Histopathology of the Skin. 6th Ed; Philadelphia: JB Lippincott, 1983:544-546.
41. Sato K, Leidal R, Sato F. Morphology and development of an apoeccrine sweat gland in human axillae. Am J Physiol.1987; 252(1 Pt 2): R166-80. [PubMed]
42. Mazoujian G, Margolis R. Immunohistochemistry of gross cystic disease fluid protein (GCDFP-15) in 65 benign sweat gland tumors of the skin. Am J Dermatopathol. 1988; 10(1): 28-35. [PubMed]
43. Ansai S, Koseki S, Hozumi Y, Kondo S. An immunohistochemical study of lysozyme, CD-15 (Leu M1), and gross cystic disease fluid protein-15 in various skin tumors: assessment of the specificity and sensitivity of markers of apocrine differentiation. Am J Dermatopathol. 1995; 17(3): 249-55. [PubMed]
44. Helwig EB, Hackney VC. Syringadenoma papilliferum; lesions with and without nevus sebaceous and basal cell carcinoma. AMA Arch Derm. 1955; 71(3): 361-72. [PubMed]
45. Fusaro RM, Goltz RW. Histochemically demonstrable carbohydrates of appendageal tumors of the skin. J Invest Dermatol. 1962; 38: 137-42. [PubMed]
46. Taylor RS, Perone JB, Kaddu S, Kerl H. Appendage tumor and hamartomas of the skin. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffel DJ (eds). Fitzpatrick’s Dermatology in General Medicine. 7th Ed; McGraw Hill Medical, 2008: 1072.
47. Jordan JA, Brown OE, Biavati MJ, Manning SC. Congenital syringocystadenoma papilliferum of the ear and neck treated with the CO2 laser. Int J Pediatr Otorhinolaryngol 1996; 38(1): 81-7. [PubMed]
© 2010 Dermatology Online Journal