Vitiligo: A good prognostic factor in melanoma?
Published Web Location
https://doi.org/10.5070/D38gs7072jMain Content
Vitiligo: A good prognostic factor in melanoma?
Daniela Cunha, Fernando Assis Pacheco, Jorge Cardoso
Dermatology Online Journal 15 (2): 15
Department of Dermatology, Hospital Curry Cabral, Lisbon, Portugal. danielaccunha@gmail.comAbstract
The association of vitiligo with immunologic therapy for melanoma is generally regarded as a good prognostic factor. Nevertheless, the immunopathogenesis of vitiligo remains incompletely understood. The authors report the case of a 71-year-old woman who had a malignant melanoma on the posterior aspect of the left leg in 1982. The patient underwent wide local excision and elective left inguinal lymphadenectomy. Sixteen years later two amelanotic malignant melanoma metastases were observed on the left inferior limb and were excised. Interferon treatment was administered for only eight months owing to side effect intolerance. Two years later, vitiligo-like macules appeared over her face and trunk. Since then, several further cutaneous metastases were found; all of them were limited to the left inferior limb. The patient remained free of visceral metastasis until December 2007, when a single lung mass was identified on a CT scan. The distinctive feature in this patient was a long survival that was free of identifiable visceral metastases. Despite the two year gap between the appearance of vitiligo and interferon treatment, the former might be indicative of a delayed immunological response against the melanoma cells, supporting the concept of a better therapeutic outcome in this cluster of patients.
Introduction
The manifestation of vitiligo has been reported in melanoma patients by several investigators [1, 2], but with different incidences. Even though the clinical association is well known, the immunopathogenesis remains incompletely understood. Epidemiological data suggest that the appearance of vitiligo at some point after immunologic therapy for metastatic melanoma correlates with a better outcome, and might be regarded as a good prognostic factor [3, 4, 5, 6, 7].
Clinical case
This report describes the case of a 71-year-old woman who had a malignant melanoma on her left leg (posterior aspect) 26 years ago (in 1982). The patient had a Fitzpatrick skin type II and no other relevant past medical history with the exception of type II diabetes mellitus. Histopathological examination of the primary excision specimen revealed a Clark IV, 4 mm Breslow deep malignant melanoma. She underwent re-excision with 2 cm margins, closed by full thickness skin grafting. Elective left inguinal lymphadenectomy was also performed and lymph nodes were negative for melanoma metastases. She had no intra- or post-surgical complications and underwent follow-up care. For the following decade, the patient had no evidence of residual disease or metastatic lesions and then she disappeared from follow-up.
Sixteen years after the initial diagnosis and surgery (in 1998), an erythematous nodule emerged and slowly grew on her left thigh. However, she only returned for consultation two years later (in 2000) when a similar lesion appeared on her left leg. Both lesions were excised and the histopathological examination was consistent with malignant melanoma metastases. An alpha 2b-interferon regimen was started (5 million U, 3 times a week), but it was stopped after 8 months due to side effects including persistent nausea, myalgia, and asthenia.
For the next two years, the patient was maintained under follow-up care and remained free of identifiable melanoma metastases. In 2002, circumscribed depigmented macules with well-defined borders appeared over her neck and right trunk (Figs. 1 & 2). These were assumed to be vitiligo-like leukoderma in the context of metastatic malignant melanoma. No treatment was undertaken and these depigmented lesions progressively enlarged, involving her face, anterior trunk, and abdomen in a bilateral asymmetrical fashion. No other signs of autoimmune disease were detected.
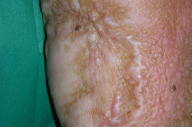 | 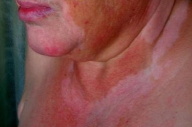 |
| Figure 1 | Figure 2 |
|---|
In 2004 (two years later), a new metastatic nodule was found on her left leg. The lesion was excised and histopathological examination confirmed malignant melanoma metastasis. Since then, multiple (10) cutaneous melanoma metastases have been found on the left inferior limb. Lesions were excised and histopathologic confirmation was performed.
For 25 years, our patient's laboratory tests have remained unremarkable (including LDH) and she has had no evidence of distant metastases in periodical thoracic, abdominal, and pelvic computed axial tomography. In December 2007, however, a routine computed tomography revealed a solid mass in the apical segment of the left lung, suggestive of a distant metastasis. In spite of this finding, the patient refused to perform further investigative procedures. She declined lung mass surgical excision or systemic chemotherapy but accepted Interferon alpha 2b treatment (3 million units, 3 times a week). This treatment was stopped 7 months later due to an accidental femoral neck fracture. Since then, the lung mass slightly enlarged (up to 14mm) but no other visceral metastases were identified.
Discussion
Vitiligo is an autoimmune cutaneous pigmentary disorder, characterized by circumscribed depigmented macules. The precise mechanism involved in the melanocyte destruction in vitiligo remains unknown; however, its frequent association with other autoimmune diseases (as autoimmune thyroiditis, lupus erythematous, and insulin-dependent diabetes mellitus) [8, 9] and the identification of specific melanocyte antibodies strongly suggests an autoimmune mechanism [1, 9].
The statistically significant association between vitiligo and melanoma is well recognized [3] and some clinical trials have identified a correlation of vitiligo with a better therapeutic outcome for patients treated with recombinant cytokines for metastatic melanoma [1, 6, 7]. The pathogenic process involved in this association is not completely understood, but it may rely on an immune response directed against melanoma-associated antigens, which are simultaneously expressed by melanoma cells and normal melanocytes (e.g., MART-1, tyrosinase, gp100, tyrosinase-related protein 1 and 2) [1]. This immune reactivity may be a response prompted by melanoma cells, leading to destruction of malignant cells and normal melanocytes, ultimately giving rise to vitiligo. Even though the mechanism remains to be clearly elucidated, available data seem to support the hypothesis of a T cell-dependent immune response [4, 10, 11, 12]. Hence, this destruction seems to result from an autoimmune mechanism triggered in response to melanoma [1].
Melanoma patient survival may rely upon different independent prognostic factors, including age and sex, anatomic site, tumor thickness and ulceration, number of positive lymph nodes, macroscopic versus microscopic lymph node involvement [13, 14, 15], and site of distant metastases [14]. In the present case, gender, age, lesion location (inferior limb), and the absence of identifiable node metastasis by histopathological examination are regarded as good prognostic factors, despite the tumor's Breslow thickness of 4 mm.
A distinctive feature in this clinical case was the patient's disease-free survival for sixteen years and an additional nine years with metastatic disease clinically limited to the inferior limb after the excision of a 4 mm Breslow thickness malignant melanoma at the age of 46 years. Despite the two year gap between alpha 2b-interferon treatments and the appearance of vitiligo, it is not clear whether the latter was the result of a delayed immunological response against melanoma cells, as seen in other cases of immunotherapy-associated vitiligo. This may immunological response may help to explain the patient's long survival and strengthen the association of a more favorable therapeutic outcome in this cluster of patients.
References
1. Boasberg P, Hoon D, Piro L et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol, 2006;126:2658-2663. [PubMed]2. Schallreuter KU, Levening C, Berger J. Vitiligo and cutaneous melanoma - A case study. Dermatologica, 1991;183:239-45. [PubMed]
3. Le Gal FA, Avril MF, Bosq J et al. Direct evidence to support the role of antigen-specific CD8+ T cells in melanoma-associated vitiligo. J Invest Dermatol, 2001;117:1464-1470. [PubMed]
4. Uchi H, Stan R, Turk MJ et al. Unraveling the complex relationship between cancer immunity and autoimmunity: lessons from melanoma and vitiligo. Adv Immunol, 2006;90:215-241. [PubMed]
5. Daneshpazhooh M, Shokoohi A, Dadban A et al. The course of melanoma-associated vitiligo: a report of a case. Melanoma Res, 2006;16(4):371-3. [PubMed]
6. Wankowicz-Kalinka A, Le Poole C, van den Wijngaard R et al. Melanocyte-specific immune response in melanoma and vitiligo: two faces of the same coin? Pigment Cell Res, 2003;16(3):254-60. [PubMed]
7. Gogas H, Ioannovich J, Dafni U et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Eng J Med, 2006;354:709-18. [PubMed]
8. Kemp EH, Waterman EA, Weetman AP. Autoimmune aspects of vitiligo. Autoimmunity 2001;34:65-77. [PubMed]
9. Ongenae K, Van Geen N, Naeyaert J. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res, 2003;16:90-100. [PubMed]
10. Lengagne R, Le Gal FA, Garcette M et al. Spontaneous vitiligo in an animal model for human melanoma: role of tumor specific CD8+ Tcells. Cancer Res, 2004;64:1496-1501. [PubMed]
11. Garbelli S, Mantonvani S, Palermo B et al. Melanocyte-specific cytotoxic T cell responses in vitiligo: the effective variant of melanoma immunity? Pigment Cell Res, 2005;18(4):234-42. [PubMed]
12. Luiten RM, Kueter EW, Gallee MP et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cell in metastatic melanoma patients. J Clin Oncol, 2005;23:8978-91. [PubMed]
13. Balch C, Soong S, Ross MI et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Ann Surg Oncol, 2000;7(2):87-97. [PubMed]
14. Balch C, Soong S, Atkins M et al. An Evidence-based Staging System for Cutaneous Melanoma. CA Cancer J Clin, 2004;54:131-149. [PubMed]
15. Dessureault S, Soong SJ, Ross MI et al. Improved staging of node-negative patients with intermediate to thick melanomas (>1 mm) with the use of lymphatic mapping and sentinel lymph node biopsy. Ann Surg Oncol 2001;8(10):766-770. [PubMed]
© 2009 Dermatology Online Journal

