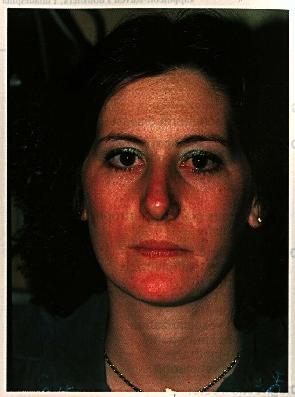
Chapter 41Acne RosaceaMarian S. Macsai, Mark J. Mannis, and Arthur C. HuntleyDISEASE ENTITY Acne rosacea is a chronic acneiform disorder affecting both the skin and the eye. It is a syndrome of undetermined etiology characterized by both vascular and papulopustular components involving the face and occasionally the neck and upper trunk. Clinical findings are usually limited to the sun exposed areas of the face and chest and include mid facial erythema, telangiectasias, papules and pustules, and sebaceous gland hypertrophy. Rosacea is characterized by episodic flushing of affected areas, which may be associated with consumption of alcohol, hot drinks, or spicy foods. During inflammatory episodes, affected areas of the skin, primarily the convexities of the face, develop swelling, papules, and pustules. The skin lesions are notable for the absence of comedones, which distinguishes this disorder from acne vulgaris. Rhinophyma is a late finding. Ocular rosacea is a term used to describe the spectrum of eye findings associated with the skin involvement. Ocular involvement may include meibomian gland dysfunction and/or chronic staphylococcal lid disease, recurrent chalazia, chronic conjunctivitis, peripheral corneal neovascularization, marginal corneal infiltrates with or without ulceration, episcleritis and iritis. Occasionally, the ocular manifestations may precede skin involvement, delaying the diagnosis. Rosacea occurs most commonly in adult life, between the ages of 30 and 60 years. It may also be found in children, although rarely (1,2). In a series of 47 patients with ocular rosacea, the decade of prevalence was 51-60 years (3). Ocular involvement occurs in more than 50% of patients (4). Women have been traditionally considered to be affected with twice the frequency of men, although some data suggests that the distribution between men and women is equal (5). Cases with ocular manifestations are about evenly divided between the sexes or show only a small female preponderance (6). The distribution of cases by age in the two sexes is similar. Both acne rosacea and ocular rosacea have been documented in blacks (7-9). Increased pigmentation in the black population may mask the early lesions of rosacea, accounting for previous failure to recognize the disease in the black population. There is a wide-spread clinical impression that rosacea mainly affects fair-skinned people of northern European descent or Celtic origin (10). However, studies have not substantiated this assumption. |
|
Dermatologic
Skin involvement in rosacea is characterized by blotchy or diffuse erythema, telangiectasias, papules, pustules, and sebaceous gland hypertrophy. The lesions are distributed across the blush areas of the face, involving the nose, cheeks, chin, and central area of the forehead, and may include the neck and chest. Initially, the hyperemia may be episodic, but within months or years it becomes chronic with the eventual development of telangiectasias, which may be fine or coarse.

Certain persons flush and develop rosaceous lesions in other areas such as the epigastrium (11). The skin frequently has an oily appearance. In most patients, asymptomatic papular, and less frequently, pustular lesions resembling acne vulgaris, may occur. Rosacea is distinguished from acne vulgaris by the absence of comedones and by its confinement to flush areas. Acne vulgaris commonly involves the back and the chest as well as the face, while rosacea is usually limited to the face. In addition, the hypertrophic changes seen in rosacea are not features of acne vulgaris (12). Rhinophyma, an irregular lobulated thickening of the skin of the nose with follicular dilatation and a purplish-red discoloration, may be a complication of long-standing involvement.
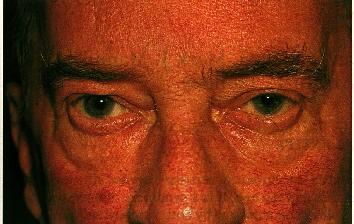
1b: Chronic, more advanced disease is characterized by increasing telangiectasia. In this patient there is also early sebaceous gland hypertrophy and rhinophyma.
Although the nose, particularly its lower portion, is the most common site of involvement, the cheeks, forehead, chin or ears may also develop tissue hypertrophy. Subjective manifestations of rosacea are minimal, although a bothersome, burning sensation may be experienced during hyperemic episodes. Like the initial hyperemic episodes, the burning may initially be transient, but within months or years may become permanent or disappear. Rosacea runs a chronic course punctuated by episodes of acute inflammation. The mean duration of the disease is between 9 and 13 years (13).
Ophthalmologic
Ocular symptoms and involvement may antedate skin involvement. In one study, 20% of patients with rosacea presented first with ocular involvement, 53% of patients developed skin lesions before eye findings appeared, and the eye and skin findings were simultaneous in 27% (6).
Ocular findings are grouped as either minor or major. Non-sight-threatening minor complications are more common (14). One study cites ocular complications of rosacea in 58% of patients and corneal involvement specifically in 33% (4). Ocular symptoms most commonly begin with foreign body sensation, pain, and burning (15). The latter is a particularly common symptom. Ocular signs include lid margin telangiectasias, chronic blepharitis, meibomitis, chalazia, papillary conjunctivitis, superficial punctate keratopathy, corneal infiltrates, and ulcerative keratitis, episcleritis, scleritis, iritis, and vitritis (14).
Lids/conjunctiva
The commonest clinical manifestation is blepharoconjuncivitis. In such cases, the lid margin demonstrates capping and inspissation of the meibomian gland orifices along with a characteristic filigree telangiectasis of the lid margin.
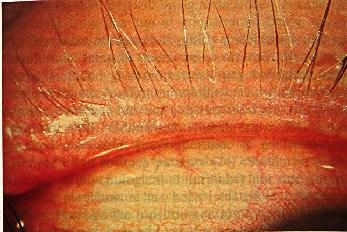
A history of recurrent hordeolum and chalazion is common. Excessive meibomian secretions may result in foamy tears. As many as 55% of patients undergoing chalazion surgery have been reported in one study to have signs of cutaneous rosacea (16). This finding emphasizes the strong association between rosacea and meibomian gland dysfunction . In addition, one may see the findings of staphylococcal blepharoconjunctivitis present concomitantly at the lid margin (17-19).
Conjunctival findings may vary. Commonly, there is diffuse hyperemia with marked congestion of the bulbar conjunctival vessels in the interpalpebral space.
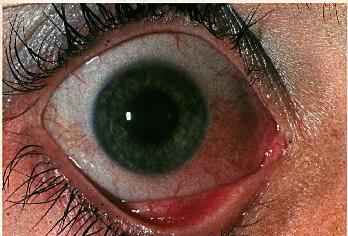
Figure 2b: Characteristic interpalpebral bulbar conjunctival injection in rosacea blepharoconjunctivitis in the patient seen in Figure 1.
There is often a follicular reaction in the inferior fornix as well as a fine, diffuse papillary hypertrophy of the tarsal conjunctiva. Although commonly bilateral, the findings in rosacea can be asymmetrical. Lemp has reported the association of keratoconjunctivitis sicca in up to 40% of patients (20).
Cornea
Rosacea keratitis represents a much more significant clinical problem. In cutaneous rosacea, corneal involvement is reported to occur in 5-30% of patients in an equal sex distribution (4,21). In contrast, in patients with frank ocular rosacea, the incidence of corneal involvement is between 75 and 85% (6,25) The inferior cornea is the usual site of rosacea keratitis. Findings may range from mild punctate epithelial keratitis accompanying the blepharoconjunctivitis to corneal vascularization, infiltration, ulceration, and perforation. In severe cases, peripheral corneal vascularization of the inferior two thirds of the cornea may progress to sterile corneal infiltration, ulceration, and rarely, perforation (22).
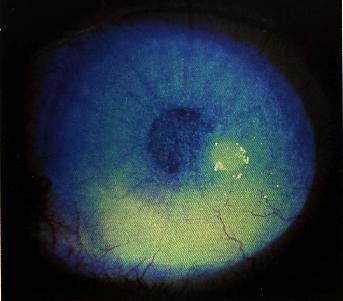
Characteristic, but not necessarily specific, "spade-like" triangular infiltrates with their base at the limbus may develop.
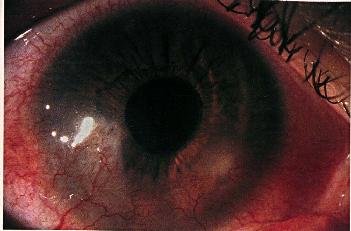
The vascularization may progress to the center of the cornea with severe disease and repeated attacks.
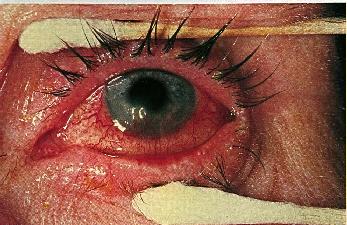
Decreased visual acuity may result from scarring and surface irregularity in more advanced cases. Episodes of corneal melting may occur at the sites of infiltrates resulting in perforation.
Other: Episcleritis (6), scleritis (23), and iritis have been reported (14,17). Diagnosis The diagnosis of acne rosacea is based exclusively on clinical findings, primarily typical skin changes. There are no available diagnostic tests. However, when ocular symptoms precede the skin changes as seen in 20% of cases, diagnosis may be difficult. The ocular changes when seen in combination with erythema and telangiectasias of the central area of the face accompanied by papules pose no diagnostic difficulty. A group of patients with ocular rosacea may have minimal objective signs despite marked symptoms requiring the ophthalmologist to rely heavily on the dermatologic findings to confirm the diagnosis.
Etiology The cause of rosacea is poorly understood, although numerous theories have been offered. Hypotheses have included gastrointestinal, psychological, infectious, climatic and immunological causes, although scientific evidence has not substantiated any of these as primary (10). Controlled studies have not demonstrated consistent preponderance of gastrointestinal symptoms in rosacea patients (24). Similarly, neither a distinct psychological abnormality nor one pharmacological mechanism has been isolated in rosacea patients. Perhaps the most commonly touted of the etiologic theories is based on the presence of Demodex folliculorum in patients with rosacea. Demodex folliculorum has been considered a causative agent of rosacea in the past (25,26). The organism feeds on sebum, and in some cases treatment of demodex infestation has noted improvement in the rosacea (14,27). However, in a review of 79 biopsies, Marks noted demodex folliculorum in only 19% of the specimens (28). A bacterial cause for the disease has been hypothesized, but no consistent findings of one bacteria have been demonstrated (5). Climate, specifically exposure to extremes of sun and cold, may have an effect on the course of the disease, but the role of climate in what appears to be a connective tissue disorder is not clear. Finally, an auto immune process has been suggested, and tissue fixed immunoglobulins have been reported in patients with chronic inflammation of rosacea, but no other evidence has been found (29). Recent experimental evidence has suggested this disease may represent a type of hypersensitivity reaction (30).
Pathogenesis The pathogenesis of rosacea is unknown, but it is presumed to be a genetically determined anomalous vascular response that develops in the third to sixth decades of life. The hypothesis, that the basic pathogenesis of the disease is a flushing disorder, is based on several findings. The disease appears to be more frequent and prevalent in northern climates where cold exposure is frequent, and in light-skinned persons in whom flushing is common. In addition, in the carcinoid syndrome, recurrent flushing over the face and upper chest has resulted in full blown rosacea (4).
An increase in solar elastosis is present in rosacea biopsy specimens (28). Pathologists hypothesize that this altered connective tissue gives inadequate support to small vessels, resulting in prolonged vasodilatation and secondary immune complex deposition. The presence of pronounced sebaceous gland hypertrophy, as seen in rhinophyma, has resulted in the hypothesis that rosacea is a sebaceous gland disease. However, sebaceous gland hypertrophy is not a typical feature of early rosacea lesions (28). On the other hand, meibomitis is a frequent accompaniment of rosacea, and a diffuse sebaceous gland abnormality has been suggested as the cause of meibomitis (31). A variety of hypotheses relating acne rosacea to an underlying gastrointestinal or psychosomatic disorder have not been supported by scientific evidence (32). No single hypothesis appears to adequately explain both the vascular changes and the inflammatory reaction seen in acne rosacea, leaving the pathogenesis unclear.
The exact pathogenesis of the corneal findings is not known, although it appears to be closely related to the lid disease. Meibomian gland dysfunction and S. aureus toxins can produce tear film instability (33). An increase in free fatty acids in the tear film may cause conjunctival hyperemia and punctate corneal epithelial breakdown. This breakdown may allow antigens--probably staphylococcal in origin--access to the corneal stroma where it combines with anti-staphylococcal antibodies (34). Antigen-antibody complexes may then activate complement through the classical pathway (C1) and generate C5a. C5a is a potent chemotactic agent for neutrophils resulting in peripheral corneal infiltration and ulceration.
Histopathology/Dermatopathology Histopathologic findings in rosacea dermatitis include vascular dilatation of the small vessels with perivascular infiltration of histiocytes, lymphocytes, and plasma cells. Dermal changes include loss of integrity of the superficial dermal connective tissue with edema, disruption of collagen fibers, and frequently severe elastosis (28). Follicular localization is infrequent and, when seen, is usually manifest clinically as pustules. However, there is no primary follicular abnormality. Rhinophyma is characterized histologically by an increase in sebaceous glands and connective tissue, follicular and vascular dilatation, edema, and a scattered infiltrate of perivascular lymphocytes and histiocytes (35). Immunoglobulin and compliment deposition at the dermal epidermal junction have been reported in conjunctival and skin biopsies from rosacea patients (36). Ocular pathologic findings include conjunctival and corneal infiltration with chronic inflammatory cells, including lymphocytes, epithelioid cells, plasma cells, and giant cells (14).
Differential Diagnosis The differential diagnosis of acne rosacea includes acne vulgaris, seborrheic dermatitis, lupus erythematosus, syphilis, tuberculosis, periorbital dermatitis, lupus malaris disseminatus, erysipelas, polymorphous light eruption, actinic reticuloid, and chronic topical corticosteroid therapy (37). A carcinoid syndrome with episodic facial flushing may resemble acne rosacea and as mentioned previously may eventually result in acne rosacea. However, the 24-hour urinary excretion of five hydroxy indoleacetic acid is normal in acne rosacea. The differential diagnosis of ocular rosacea includes staphylococcal and seborrheic blepharokeratoconjunctivitis and sebaceous gland carcinoma.
MANAGEMENT
Dermatologic Disease
The outcome of a successful management regimen is usually control rather than eradication of the disease. Advising the patient to avoid those stimuli that tend to exacerbate the disease--exposure to extremes of heat and cold, excessive sunlight, ingestion of hot liquids, alcohol, and spicy foods--may help. Although its mechanism of action is not clearly understood, the mainstay of treatment is the use of oral tetracycline, especially for the papular or pustular lesions (38-40). The dosage utilized is generally 250 mg every 6 hours for the first 3 to 4 weeks, followed by tapering based on clinical response. Doxycycline and minocycline (50-100 mg every 12 hours) are also effective and have the advantage of less frequent dosage and less concern over problems with gastrointestinal absorption. Patients who are intolerant to the tetracyclines may benefit from the use of erythromycin. Oral isotretinoin, in doses similar to those used for acne vulgaris, has also been effective for the inflammatory lesions, erythema, and rhinophyma (41,42). There is, however, no beneficial effect on the telangiectasias and isotretinoin may cause blepharitis and conjunctivitis (43). Other oral agents that have been used include ampicillin and metronidazole. Clonidine may also be of some value in reducing facial flushing (44).
Topical therapy for rosacea is generally less successful than systemic treatment. Metronidazole may be effective topically (45,46). It is available commercially as a 0.75% gel and, when applied twice daily, substantially reduces inflammatory lesions (47). Although topical corticosteroids may effectively improve signs and symptoms, long-term therapy is not advisable since it may cause atrophy, chronic vasodilation, and telangiectasia formation (48).
The treatment of chronic skin changes may require surgical intervention. Telangiectasias may be treated by electrocautery or using the tunable dye laser (49). Severe rhinophyma is treated by paring with a scalpel, excision with skin grafting, dermabrasion, bipolar electrocautery, or by means of the argon or carbon dioxide laser (50-53).
Ophthalmologic Disease Management of ocular rosacea varies to some extent depending on the clinical manifestations. Nonetheless, systemically administered antibiotics remain the primary form of therapy along with aggressive lid hygiene. Both tetracycline and doxycycline have been studied clinically in the treatment of rosacea eye disease (15,54-56). In a recent study of 24 patients with ocular rosacea diagnosed by both an ophthalmologist and a dermatologist, both tetracycline hydrochloride and doxycycline proved effective in the management of ocular rosacea. In this study, tetracycline alleviated symptoms faster, while doxycycline had the advantage of easier compliance and tended to cause fewer gastrointestinal side-effects (55). As for the skin manifestations, the precise mechanism of action of tetracycline in control of this disease is not clear although several hypotheses have been entertained (57). As in the management of the cutaneous disease, the usual tetracycline dosage is 250 mg four times a day for three weeks initially with tapering thereafter based on clinical response (15) The dosage of doxycycline is generally 50-100 mg twice daily initially with subsequent tapering. With the exception of corneal neovascularization and healed scarring, all signs of rosacea generally respond to tetracycline beginning within two weeks after initiation of therapy (56) Cessation of systemic therapy may be accompanied by relapses, and many patients require long-term, maintenance therapy.
Adjunct therapy to systemic antibiotics is important in the management of this disorder. Intensive lid hygiene with warm soaks, dilute baby shampoo, or commercially available eye scrubs will help to manage the blepharitis. The importance of these local measures cannot be overemphasized. Some clinicians recommend the use of a bacteriostatic ointment once daily at bedtime in addition to systemic antibiotics and lid hygiene. The value of this topical ointment has not been verified in clinical studies. However, it does encourage patients to perform thorough lid hygiene in the morning to remove residual ointment.
Although the use of corticosteroids can be hazardous in patients with rosacea, (58) , if judiciously applied, these agents can be useful in the severe inflammatory component of the blepharitis as well as the episcleritis, keratitis, and iritis (14) . Patients must, however, be monitored closely, and infectious keratitis must always be ruled out prior to the use of topical steroids.
Surgical intervention may be warranted in rosacea keratitis in the case of impending or frank perforation secondary to keratitis with corneal melting.This may include conjunctival flaps, tectonic lamellar keratoplasty, or penetrating keratoplasty. In addition, scarring left by the disease may the indication for optical keratoplasty. The latter should be undertaken only if the surface and lid inflammatory disease is under control. Because of the surface disease in these patients, they are at higher risk both for post-keratoplasty surface problems as well as immune graft rejection.
REFERENCES 1. Savin J, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
2. Stevens G, Lemp M. Acne rosacea. In: T Weingeist , D Gould. The Eye in Systemic Disease. Philadelphia: Lippincott, 1990.
3. Jenkins MS, Brown SI, Lempert SL, Weinberg RJ. Ocular rosacea. In: BD Srinivasan. Ocular therapeutics. New York: Masson Publishing, 1980.
4. Starr PAH, McDonald A. Oculocutaneous aspects of rosacea. Proc R Soc Med. 1969;62:9.
5. Marks R. Concepts in the pathogenesis of rosacea. Br J Dermatol. 1968;80:170.
6. Borrie P. Rosacea with special reference to its ocular manifestations. Br J Ophthalmol. 1953;65:458.
7. Brauner GJ. Cutaneous disease in black races. In: SL Moschella , HJ Hurley. Dermatology. Philadelphia: W.B. Saunders, 1985.
8. Browning DJ, Rosenwasser G, Lugo M. Ocular rosacea in blacks. Am J Ophthalmol. 1986;101:441-444.
9. Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
10. Marks R. Rosacea, flushing and perioral dermatitis. In: RH Champion, JL Burton , FJG Ebling. Textbook of Dermatology. London: Blackwell Scientific Publications, 1993.
11. Wilkin JK. Epigastric rosacea. Arch Dermatol. 1980;116:584.
12. Marks R, Jones EW. Disseminated rosacea. Br J Dermatol. 1969;81:16.
13. Irvine C, Marks R. Prognosis and prognostic factors in rosacea. In: R Marks , G Plewig. Acne and related disorders. London: Martin Dunitz, 1989.
14. Browning DJ, Proia AD. Ocular Rosacea. Surv Ophthalmol. 1986;31:145-158.
15. Jenkins MA, Brown SI, Lempert SL, al. e. Ocular rosacea. Am J Ophthalmol. 1979;88:618-622.
16. Lempert SL, Jenkins MS, Brown SI. Chalazia and rosacea. Arch Ophthalmol. 1979;97:1652.
17. Wise G. Ocular rosacea. Am J Ophthalmol. 1943;26:591-609.
18. Doggart JH. The ocular complications of acne rosacea. Br J Ophthalmol. 1931;15:446-457.
19. Foster CS. Ocular surface manifestations of neurological and systemic disease. Int Ophthalmol Clin. 1979;69:207-242.
20. Lemp M, Mahmood MA, Weiler HH. Association of rosacea in keratoconjunctivitis sicca. Arch Ophthalmol. 1984;102:556.
21. Thygeson P. Dermatoses with ocular manifestations. In: A Sorsby. Systemic ophthalmology. London: Butterworth, 1951.
22. Goldsmith AJB. The ocular manifestations of rosacea. Br J Dermatol. 1953;
23. Richter S. Skleraperforation bei rosaceokeratitis. Klin Monatsbl Augenheillkd. 1965;146:422-424.
24. Rebora A. Rosacea. J Invest Dermatol. 1987;88:56s-60s.
25. Spickett P. Aetiology of rosacea. Br Med J. 1962;i:1625-1626.
26. Russell BF. Some aspects of the biology of the epidermis. Br Med J. 1962;i:815-820.
27. Rufli T, Buchner SA. T-cell subsets in acne rosacea lesions and the possible role of demodex folliculorum. Dermatologica. 1984;169:1.
28. Marks R, Harcourt-Webster JN. Histopathology of rosacea. Arch Dermatol. 1969;100:682.
29. Nunzi E, Rebora A, Hamerlinck F, al. e. Immunopathological studies on rosacea. Br J Dermatol. 1980;103:543.
30. Marks R. Histogenesis of the inflammatory component of rosacea. Proc Roy Soc Med. 1973;66:742-745.
31. McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977;84:788.
32. Wilkin JK. Rosacea. Int J Dermatol. 1983;22:343.
33. Smolin G, Okumoto M. Staphylococcal blepharitis. Arch Ophthalmol. 1977;95:812-816.
34. Mondino BJ. Inflammatory diseases of the peripheral cornea. Ophthalmol. 1988;95:463-472.
35. Pochi PE. Acne rosacea. In: DJ Demis. Clinical Dermatology. Philadelphia: J.B. Lippincott Company, 1991.
36. Manna V, Marks R, Holt P. Involvement of immune mechanisms in the pathogenesis of rosacea. Br J Dermatol. 1982;107:203.
37. Leyden JJ, Thew M, Klingman AM. Steroid rosacea. Arch Dermatol. 1974;110:619.
38. Sneddon I, 1966. A clinical trial of tetracycline in rosacea. Br J Dermatol. 1966;78:649-653.
39. Wereide K. Long tern treatment of rosacea with oral tetracycline. Acta Derm Venereol. 1969;49:176-179.
40. Marmion VJ. Tetracyclines in the treatment of ocular rosacea. Proc Roy Soc Med. 1969;62:11-12.
41. Shalita AR, Cunningham WJ, Leyden JJ. Isotretinoin treatment of acne and related disorders: an update. J Am Acad Dermatol. 1983;9:629-683.
42. Plewig G, Nikolowski J, Wolff H. Action of isotretinoin in acne rosacea and gram negative foliculitis. J Am Acad Dermatol. 1982;6:7676-785.
43. Hoting E, Paul E, Plewig G. Treatment of rosacea with isotretinoin. Int J Dermatol. 1986;25:660-663.
44. Cunliffe JW, Dodman B, Binner JG. Clonidine and facial flushing in rosacea. Br Med J. 1977;1:105. 45. Nielsen PG. A double-blind study of 1% metronidazole cream versus systemic oxytetracycline therapy for rosacea. Br J Dermatol. 1983;109:63.
46. Bleicher PA, Charles JG, Sober AJ. Topical metronidazole therapy for rosacea. Arch Dermatol. 1987;123:609-614.
47. Aronson IK, Rumsfeld JA, West DP, al. e. Evaluation of topical metronidazole gel in acne rosacea. Drug Intell Clin Pharmacol. 1987;21:346-351.
48. Sneddon I. Adverse effect of topical fluorinated corticosteroids in rosacea. Br Med J. 1969;1:671.
49. Polla LL, Tan OT, Garden JM, al. e. Tunable pulsed dye laser for the treatment of benign cutaneous vascular ectasia. Dermatologica. 1987;174:11-17.
50. Pastorek MJ. The management of rhinophyma. Otolaryngol Clin North Am. 1972;5:639-646.
51. Verde SF. How we treat rhinophyma. J Dermatol Surg Oncol. 1980;6:560.
52. Henning JPH, Gemert MJC. Rhinophyma treated by argon laser. Lasers Surg Med. 1983;2:211.
53. Shapshay SM, al. e. Removal of rhinophyma with the carbon dioxide laser. Arch Otolaryngol. 1980;106:257.
54. Frucht-Pery, Chayet AS, Feldman ST, Lin S, Brown SI. The effect of doxycycline on ocular rosacea. Am J Ophthalmol.1989;107:434-435.
55. Frucht-Pery J, Sagi E, Hemo I, Ever-Hadani P. Efficacy of doxycyline and tetracycline in ocular rosacea. Am J Ophthalmol. 1993;116:88-92.
56. Bartholomew RS, Reid BJ, MacDonald M, Galloway NR. Oxytetracycline in the treatment of ocular rosacea: a double-blind trial. Br J Ophthalmol. 1982;66:386.
57. Salamon SM. Tetracyclines in ophthalmology. Surv Ophthalmol. 1985;29:265-275.
58. Hyndiuk RA, Chin GN. Corticosteroid therapy in corneal disease. Int Ophthalmol Clin. 1973;13:103-123.
© 1996 by Lippincott-Raven Publishers. All rights reserved. No part of this chapter may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronical, mehanical, photocopying, or recording, or otherwise, without prior written permission of the publisher.